
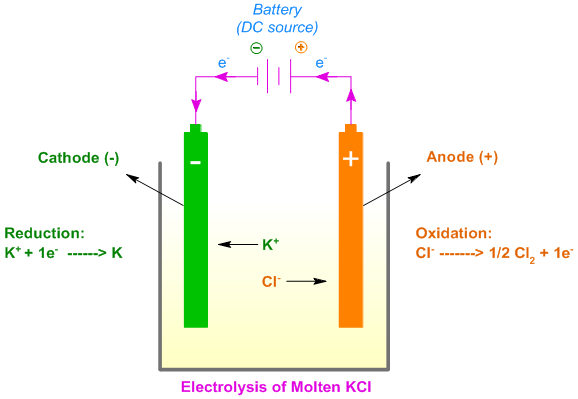
Google has not performed a legal analysis and makes no representation as to the accuracy of the date listed.) Filing date Publication date Application filed by Wuhan University WHU, Electric Power Research Institute of Guangdong Power Grid Co Ltd filed Critical Wuhan University WHU Priority to CN201410351481.0A priority Critical patent/CN104152913A/en Publication of CN104152913A publication Critical patent/CN104152913A/en Status Pending legal-status Critical Current LinksĢ. Original Assignee Wuhan University WHU Electric Power Research Institute of Guangdong Power Grid Co Ltd Priority date (The priority date is an assumption and is not a legal conclusion. More detailed, reactions 2 and 3 show the main anode and cathode reactions of chloride oxidation and hydrogen evolution, respectively. Google has not performed a legal analysis and makes no representation or warranty as to the accuracy of the list.)Įlectric Power Research Institute of Guangdong Power Grid Co Ltd Electrode Reactions and Chlorate Chemistry Main Reactions Industrial chlorate electrolysis takes place in undivided cells, where sodium chlorate and hydrogen gas are formed as described by reaction 1. List - IList - II(P)Very dilute solution of HCl(1)O2evolved at anode(Q)Very dilute solution of KCl(2)H2evolved at cathode(R)Concentrated solution of. (Express your answers as chemical equations. + 2 Kal 2 KCL 2 Draw galvamc cells based on the nct ionic equations fox the. B) Write an equation for the half-reaction that occurs in the anode in the electrolysis of molten KCl2. In the cathode reaction, the salt bridge is broken and the atoms in CrIC. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.) Pending Application number CN201410351481.0A Other languages Chinese ( zh) A) Write an equation for the half-reaction that occurs at the cathode in the electrolysis of molten KCl2.
#Cathode reaction of kcl2 pdf
Google Patents Cathodic protection device of smart sacrificial anode suitable for application in freshwaterĭownload PDF Info Publication number CN104152913A CN104152913A CN201410351481.0A CN201410351481A CN104152913A CN 104152913 A CN104152913 A CN 104152913A CN 201410351481 A CN201410351481 A CN 201410351481A CN 104152913 A CN104152913 A CN 104152913A Authority CN China Prior art keywords sacrificial anode magnet protection device cathodic protection cathodic Prior art date Legal status (The legal status is an assumption and is not a legal conclusion. Reactants Potassium - K Kalium Element 19 K Molar Mass K Oxidation Number Chlorine - Cl Element 17 Cl Molar Mass Cl Oxidation Number Products KCl2 Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The influence of cathode materials on the U electrorefining process is examined using electrochemical measurements and SEM-EDX observations. Google Patents CN104152913A - Cathodic protection device of smart sacrificial anode suitable for application in freshwater True False Electrolysis of molten KCl, At anode, 2ClCl2+2e at cathode. Sn 2+ (aq) +2e - → Sn (s) with SRP E o = -0.137 V (Cathode where reduction happens)Ģ.CN104152913A - Cathodic protection device of smart sacrificial anode suitable for application in freshwater To prevent the dissolution of the cathode material, new cathodes have been proposed based on metal oxychloride salts 51, which are more stable because the. 2H H 2(g) So the net result is that at the anode chlorine gas is released, at the cathode hydrogen gas is released, and a. The primary cathodic reaction in cryolite-based melts is the reduction of Al 3 + containing species. 2Cl Cl2(g) At the other side (cathode): K+ +e K.
\) for the voltaic cell formed by each reaction.ġ.a) Ba 2+ (aq) → Ba (s) + 2e- with SRP (for opposite reaction) E o = -2.92 V (Anode where oxidation happens)Ĭu 2+ (aq) + 2e- → Cu (s) with SRP E o = +0.340 V (Cathode where reduction happens)ġ.b) Al 3+ (aq) → Al (s) + 3e - with SRP (for opposite reaction) E o = -1.66 V (Anode where oxidation happens) The solution may be represented by K+(aq) and Cl(aq) At the positive electrode (anode) the following happens: ClCl +e.


 0 kommentar(er)
0 kommentar(er)
